DRG Successful Exhibit at ENDO Expo 2016!
DRG International, Inc. recently exhibited at ENDO EXPO 2016, the largest meeting of endocrine practitioners and researchers, from April 1 – 3 at the Boston Convention & Exhibition Center in Boston, MA. A highlight at the show was DRG’s new Hepcidin-25 Bioactive High-Sensitive (HS) kit, EIA5782, which is now for sale. You can learn more about our Hepcidin product here.

Michael V. Martini III, Director of Sales & Marketing, at the DRG International, Inc. Booth.
In addition to the presentation of several new assays DRG offered live demonstrations of our fully automated continuous access analyzer: DRG:HYBRiD-XL®. Visitors from around the world gathered to receive personalized presentations of the Hybrid-XL instrument. DRG’s Salivary Assay ELISA product line was also a big attraction at the conference, especially as DRG is preparing to launch a new, re-optimized line of salivary assays. You can find more information on DRG Salivary Assay ELISA kits here.
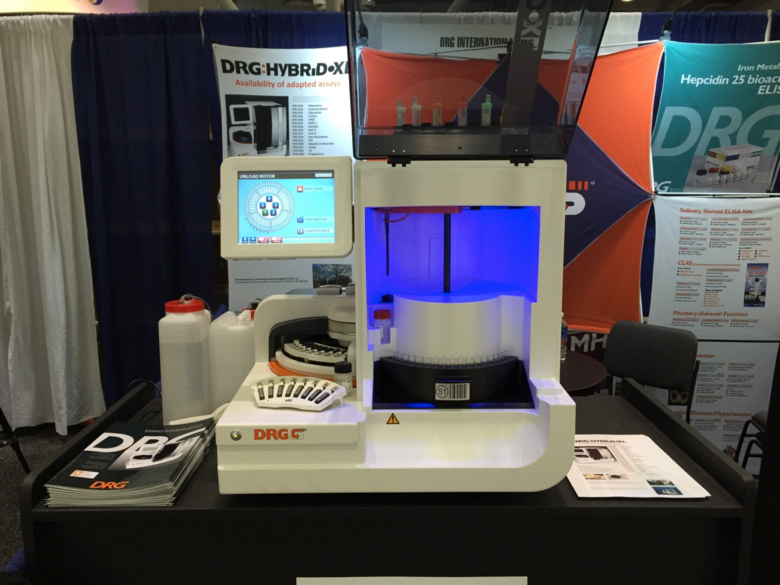
A DRG:HYBRiD.XL Fully Automated Immunoassay and Clinical Chemistry Analyzer at the DRG Booth
[starbox]
News and Updates
Parasites & Helicobacter Pylori in Egyptian Children | Calprotectin- DRG:HYBRiD-XL
Parasites & Helicobacter Pylori in Egyptian Children with or without Diabetes with Gastrointestinal Manifestations & High Calprotectin Level
Estimating the Burden of Iron Deficiency
Background: Iron deficiency (ID) is a major public health burden in African children and accurate prevalence Abstract Background and Objective Iron deficiency (ID) is a major public health burden in African children and accurate prevalence estimates are...
Early postnatal hypoferremia in low birthweight, preterm babies
Early postnatal hypoferremia in low birthweight and preterm babies: A prospective cohort study in hospital-delivered Gambian neonates Abstract Background Neonates, particularly those born preterm (PTB) and with low birthweight (LBW), are especially susceptible...