Parasites & Helicobacter Pylori in Egyptian Children | Calprotectin- DRG:HYBRiD-XL
Parasites & Helicobacter Pylori in Egyptian Children with or without Diabetes with Gastrointestinal Manifestations & High Calprotectin Level
Estimating the Burden of Iron Deficiency
Background: Iron deficiency (ID) is a major public health burden in African children and accurate prevalence Abstract Background and Objective Iron deficiency (ID) is a major public health burden in African children and accurate prevalence estimates are...

Early postnatal hypoferremia in low birthweight, preterm babies
Early postnatal hypoferremia in low birthweight and preterm babies: A prospective cohort study in hospital-delivered Gambian neonates Abstract Background Neonates, particularly those born preterm (PTB) and with low birthweight (LBW), are especially susceptible...

Hepcidin exerts negative immunological effect in tuberculosis
Hepcidin exerts a negative immunological effect in pulmonary tuberculosis without HIV co-infection, prolonging the time to culture-negative Highlights In contrast to HIV-related tuberculosis, evaluations of the influence of hepcidin in non-HIV-related...

Hepcidin 25 HS & HIV Antiretroviral Therapy
Hepcidin 25 HS & HIV Antiretroviral Therapy Study High levels of serum IL-6 and serum hepcidin and low CD4 cell count were risk factors of anemia of chronic disease in HIV patients on the combination of antiretroviral therapy. Abstract Purpose: This...
Testosterone Administration During Energy Deficit Suppresses Hepcidin and Increases Iron Availability for Erythropoiesis
Testosterone Administration During Energy Deficit Suppresses Hepcidin and Increases Iron Availability for Erythropoiesis Context: Severe energy deprivation markedly inhibits erythropoiesis by restricting iron availability for hemoglobin synthesis. Objective:...
Role of Hepcidin to Identify the Type of Anemia in Chronic Kidney Disease in Children
Role of Hepcidin to Identify the Type of Anemia in Chronic Kidney Disease in Children See full study: https://iopscience.iop.org/article/10.1088/1742-6596/1246/1/012023/meta Abstract Chronic kidney disease (CKD) may present with anemia of chronic disease (ACD),...
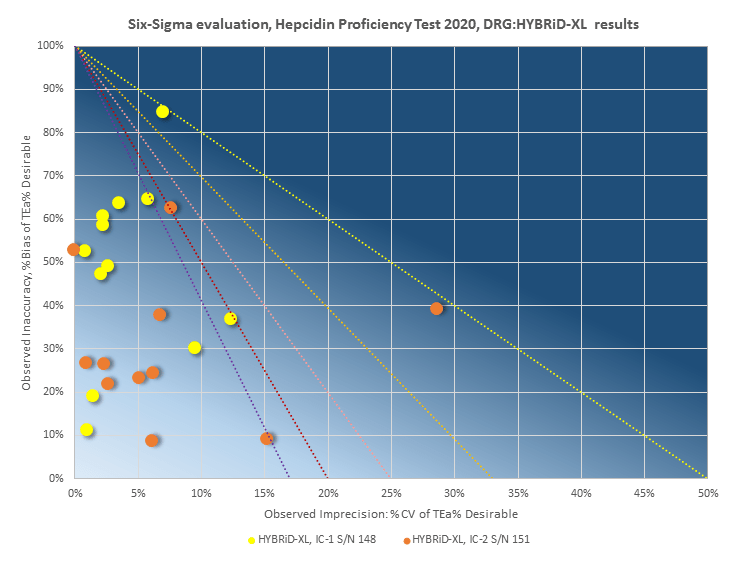
Hepcidin 25 Proficiency Test
On April 30th, 2020, an organized study by Radboud University Medical Center in Nijmegen, Netherlands, has completed a final report for the DRG International’s first ever Hepcidin Proficiency Test.
Can hepcidin be a useful marker in the diagnosis and monitoring of De Quervain thyroiditis?
The median of Hepcidin 25 serum concentration was markedly elevated in the De Quervain thyroiditis patients in comparison to control subjects and decreased significantly after therapy.
In Vitro Diagnostic Assays
The most prominent IAs are automated chemiluminescent IA (CLIA), manual ELISA, and rapid lateral flow IA (LFIA), which detect the immunoglobulin M (IgM) and immunoglobulin G (IgG) produced in persons in response to SARS-CoV-2 infection …
Click the link to learn more about how DRG is certified in the manufacturing and distribution of in-vitro diagnostic reagents used in the diagnosis of autoimmune status, cancer, cardiac markers, disease status, fertility testing, pregnancy testing, diabetes and immune status.

Click the link to learn more about how DRG is certified in the manufacturing and distribution of in-vitro diagnostic reagents used in the diagnosis of autoimmune status, cancer, cardiac markers, disease status, fertility testing, pregnancy testing, diabetes and immune status.

