Hepcidin Proficiency Test
On April 30th, 2020, we received the final report of the first ever Hepcidin Proficiency Test. This study has being organized by Radboud University Medical Center (in Nijmegen, The Netherlands) in collaboration with SKML (the Dutch Foundation for Quality Assessment in Medical Laboratories), and involved all the actual methods for Hepcidin testing, from Mass Spectrometry to ELISA and the only automation in the world for this parameter: DRG: HYBRiD-XL, HYE-5769 Hepcidin 25 (bioactive).
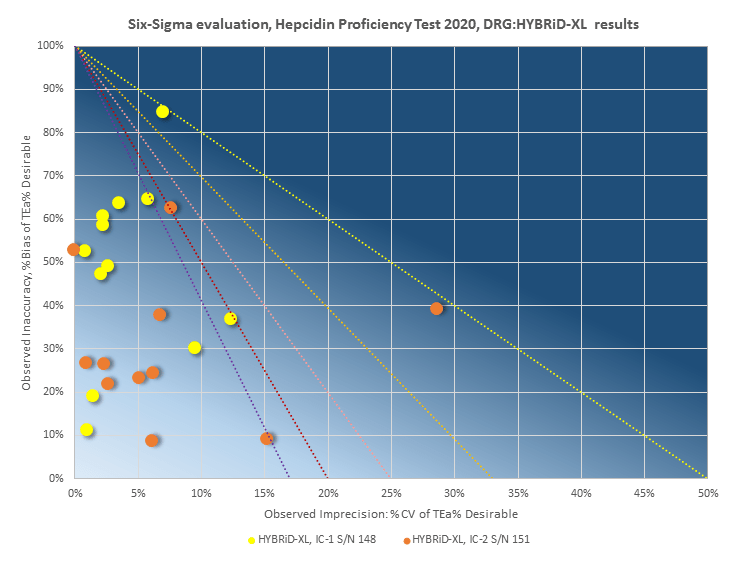
This proficiency trial involved 19 laboratories worldwide (from Australia and Japan to Germany and The Netherlands) provided independent data and proved consistent precision and accuracy of the Hepcidin results generated by two different DRG: HYBRiD-XL instruments vs Mass Spectrometry reference method.
Enjoy the (first ever) Sigma-metrics applied to Hepcidin 25 (bioactive) testing, performed on 12 x 2 “blind” samples of the independent proficiency test organized for this assay, on two DRG: HYBRiD-XL machines, against Mass Spectroscopy. In comparison with the reference Mass Spectrometry method used by HPT2020, both DRG:HYBRiD-XL instruments responded similarly to the proficiency samples (reference and patient) with a consistent precision and accuracy for all the Hepcidin 25 (bioactive) results generated.
D-dimer for DRG: HYBRiD-XL is available again.
The new lot is on stock and is ready to be ordered. Elevated levels of D-dimer are found in clinical conditions such as vein thrombosis, pulmonary embolism and disseminated intravascular coagulation. The DRG: HYBRiD-XL D-dimer Kit is an immunoturbidimetric assay.
Call Us Now! A Representative Is Standing By To Help.

Parasites & Helicobacter Pylori in Egyptian Children | Calprotectin- DRG:HYBRiD-XL
Parasites &...
Testosterone Administration During Energy Deficit Suppresses Hepcidin and Increases Iron Availability for Erythropoiesis
Testosterone...
Role of Hepcidin to Identify the Type of Anemia in Chronic Kidney Disease in Children
Role of Hepcidin to...
In Vitro Diagnostic Assays
In Vitro Diagnostic...
American Association of Clinical Chemistry Directory
American Association of...
DRG: HYBRiD-XL in Kyrgyzstan
Photo (right to...

