DRG Successful Exhibit at ENDO Expo 2016!
DRG International, Inc. recently exhibited at ENDO EXPO 2016, the largest meeting of endocrine practitioners and researchers, from April 1 – 3 at the Boston Convention & Exhibition Center in Boston, MA. A highlight at the show was DRG’s new Hepcidin-25 Bioactive High-Sensitive (HS) kit, EIA5782, which is now for sale. You can learn more about our Hepcidin product here.

Michael V. Martini III, Director of Sales & Marketing, at the DRG International, Inc. Booth.
In addition to the presentation of several new assays DRG offered live demonstrations of our fully automated continuous access analyzer: DRG:HYBRiD-XL®. Visitors from around the world gathered to receive personalized presentations of the Hybrid-XL instrument. DRG’s Salivary Assay ELISA product line was also a big attraction at the conference, especially as DRG is preparing to launch a new, re-optimized line of salivary assays. You can find more information on DRG Salivary Assay ELISA kits here.
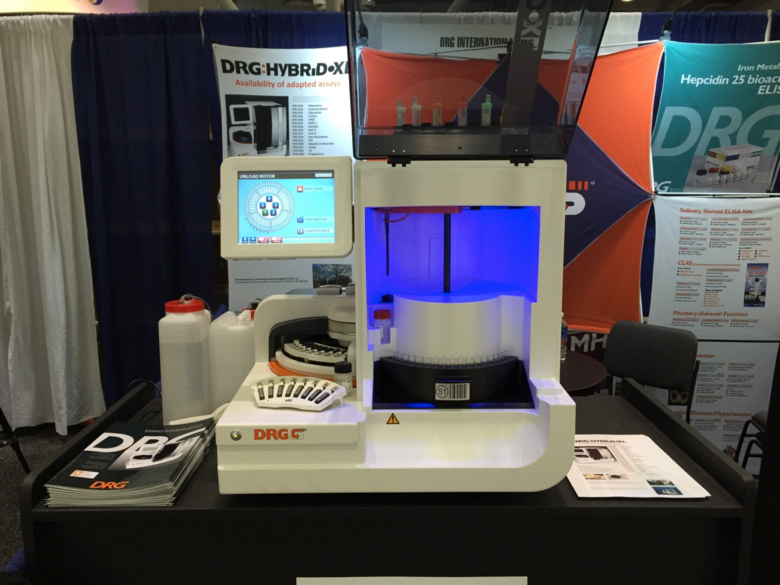
A DRG:HYBRiD.XL Fully Automated Immunoassay and Clinical Chemistry Analyzer at the DRG Booth
[starbox]
News and Updates
BioCheck Inc Acquires DRG International Inc
Transformative deal significantly increases IVD development, manufacturing and sales channel for novel ELISA and Chemiluminescent immunoassay platforms.
Parasites & Helicobacter Pylori in Egyptian Children | Calprotectin- DRG:HYBRiD-XL
Parasites & Helicobacter Pylori in Egyptian Children with or without Diabetes with Gastrointestinal Manifestations & High Calprotectin Level
Cortisol & Adrenal Androgens as Independent Predictors
Cortisol and adrenal androgens as independent predictors of mortality in septic patients. Abstract Background and Objective To determine the prognostic value of cortisol, Dehydroepiandrosterone (DHEA) and Dehydroepiandrosterone-sulfate (DHEAS), together with their...