Now Available Through
DRG International
Introducing the first of two SARS-CoV-2 Antibody Assays† developed, manufactured and distributed by DRG International for Non-US Customers Only.
Order Now!
Introducing the first of two SARS-CoV-2 Antibody Assays† developed, manufactured and distributed by DRG International for Non-US Customers Only.
SARS-CoV-2 (RBD) Total Ab ELISA†
ELISA for the qualitative and highly sensitive detection of total (IgA, IgM and IgG) antibodies against Coronavirus SARS-CoV-2 in serum and plasma.
SARS-CoV-2 (RBD) IgG ELISA†
ELISA for the quantitative and highly specific detection of human IgG antibodies against Coronavirus SARS-CoV-2 in human serum and plasma.
SARS-CoV-2 (RBD) Total Ab ELISA†
ELISA for the qualitative and highly sensitive detection of total (IgA, IgM and IgG) antibodies against Coronavirus SARS-CoV-2 in serum and plasma.
SARS-CoV-2 (RBD) IgG ELISA†
ELISA for the quantitative and highly specific detection of human IgG antibodies against Coronavirus SARS-CoV-2 in human serum and plasma.
Benefits at a Glance†
- Domain (RBD) of spike protein of SARS-CoV-2†
- High Sensitivity and Specificity
- Monitor Vaccination Success/Efficiency
- Results in 75 (Total Ab) / 105 (IgG) minutes
- Ready-to-use reagents included
- Tests are prepared for automation on open ELISA platforms
- Developed, validated and manufactured in Marburg, Germany
- EU: CE / IVD
ELISA for the qualitative and highly
sensitive detection of total
(IgA, IgM and IgG) antibodies
against Coronavirus SARS-CoV-2
in serum and plasma.
Benefits at a Glance†
- Domain (RBD) of spike protein of SARS-CoV-2†
- High Sensitivity and Specificity
- Monitor Vaccination Success/Efficiency
- Results in 75 (Total Ab) / 105 (IgG) minutes
- Ready-to-use reagents included
- Tests are prepared for automation on open ELISA platforms
- Developed, validated and manufactured in Marburg, Germany
- EU: CE / IVD
DRG SARS-CoV-2 (RBD) Total Ab ELISA† – EIA-6154
ELISA for the qualitative and highly sensitive detection of total (IgA, IgM and IgG) antibodies against Coronavirus SARS-CoV-2 in serum and plasma.
The DRG SARS-CoV-2 (RBD) total Ab ELISA† is an enzyme immunoassay for the qualitative in vitro diagnostic measurement of IgG/IgM/IgA antibodies to SARS-CoV-2 in serum and plasma (EDTA, Li-heparin or citrate).
This specific ELISA is intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection. It can support the diagnosis of COVID-19 disease and supplement direct pathogen detection via RT-PCR or pulmonary CT-Scans. In addition, serology can help to gather epidemiological information on the disease prevalence.
Assay Principle: Double-Antigen Bridging Format
Objective: Qualitative Detection of IgG, IgM, IgA
The DRG SARS-CoV-2 (RBD) Total Ab ELISA† in vitro diagnostic measurement of
the novel coronavirus that causes COVID-19
Catalog Number: (EIA-6154)
Assay Characteristics
- Diagnostic Sensitivity: 100 % (for PCR-confirmed samples)
- Method Comparison: 100% agreement to Roche Elecsys Anti-SARS-CoV-2
- Diagnostic Specificity: 99.7 %
- Intra-Assay Precision: 3.9 – 9.1%
- Inter-Assay Precision: 8.4 – 12.0%
- Excellent Linearity of Dilution: y = 1.004+1.4; R2= 0.994
- Sample Matrix: Serum or Plasma (EDTA, lithium heparin or citrate plasma)
- Total Assay Time: 75 minutes; short hands-on time
- No dilution of samples necessary
- Negative, Cut-off and Positive Control included
- All reagents ready-to-use (except Wash Solution)
- Validated according to CLSI Guidelines
- EU: CE / IVD
- This ELISA is validated for human samples, but could also be used for veterinary applications
Assay Characteristics
- Diagnostic Sensitivity: 100 % (for PCR-confirmed samples)
- Method Comparison: 100% agreement to Roche Elecsys Anti-SARS-CoV-2
- Diagnostic Specificity: 99.7 %
- Intra-Assay Precision: 3.9 – 9.1%
- Inter-Assay Precision: 8.4 – 12.0%
- Excellent Linearity of Dilution: y = 1.004+1.4; R2= 0.994
- Sample Matrix: Serum or Plasma (EDTA, lithium heparin or citrate plasma)
- Total Assay Time: 75 minutes; short hands-on time
- No dilution of samples necessary
- Negative, Cut-off and Positive Control included
- All reagents ready-to-use (except Wash Solution)
- Validated according to CLSI Guidelines
- EU: CE / IVD
- This ELISA is validated for human samples, but could also be used for veterinary applications
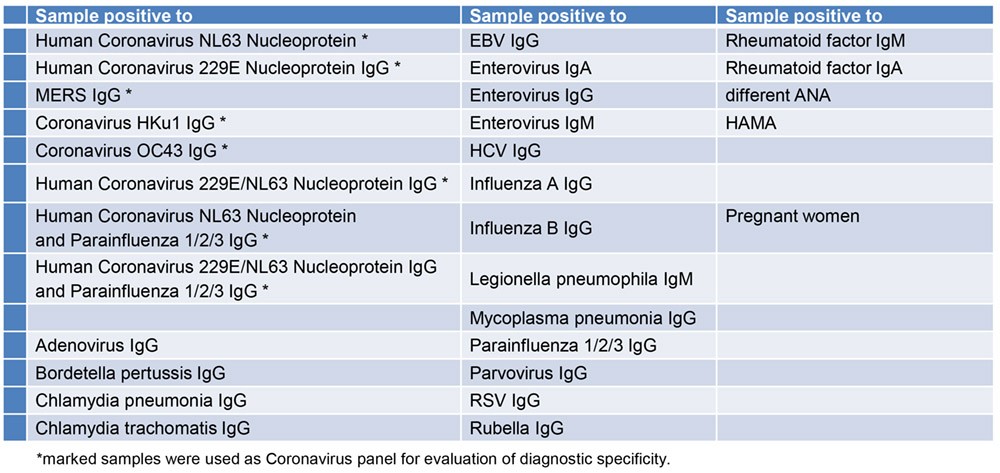
Specificity of Antigen (Cross-Reactivity)
The following samples were tested for cross-reactivity of the assay:
Specificity of Antigen (Cross-Reactivity)
The following samples were tested for cross-reactivity of the assay:
The DRG SARS-CoV-2 (RBD) Total Ab ELISA† shows no cross-reactivity to the evaluated antibodies.
SARS-CoV-2 (RBD) IgG ELISA† – EIA-6150
ELISA for the quantitative and highly specific detection of human IgG antibodies against Coronavirus SARS-CoV-2 in human serum and plasma.
Assay Characteristics
- Diagnostic Sensitivity: 100 % (for PCR-confirmed samples)
- Method Comparison: 100% agreement to Roche Elecsys Anti-SARS-CoV-2
- Diagnostic Specificity: 99.7 %
- Intra-Assay Precision: 3.9 – 9.1%
- Inter-Assay Precision: 8.4 – 12.0%
- Excellent Linearity of Dilution: y = 1.004+1.4; R2= 0.994
- Sample Matrix: Serum or Plasma (EDTA, lithium heparin or citrate plasma)
- Total Assay Time: 75 minutes; short hands-on time
- No dilution of samples necessary
- Negative, Cut-off and Positive Control included
- All reagents ready-to-use (except Wash Solution)
- Validated according to CLSI Guidelines
- EU: CE / IVD
- This ELISA is validated for human samples, but could also be used for veterinary applications.
Assay Characteristics for
EIA-6150
- Diagnostic Sensitivity: 95.2 %
- Diagnostic Specificity: 100 %
- Intra-Assay Precision: 5.4- 7.4%
- Inter-Assay Precision: 7 .0 – 13.4%
- Excellent Linearity of Dilution: y = 1.007+0.065; R2= 0.999
- Sample: 10µL Serum or Plasma (EDTA, lithium heparin or citrate plasma), 1+100 with Sample Diluent
- Total Assay Time: 105 minutes
- 4 Calibrators, Negative and Positive Control included
- All reagents ready-to-use (except Wash Solution)
- Good Assay Performance (validated according to CLSI Guidelines)
- EU: CE / IVD
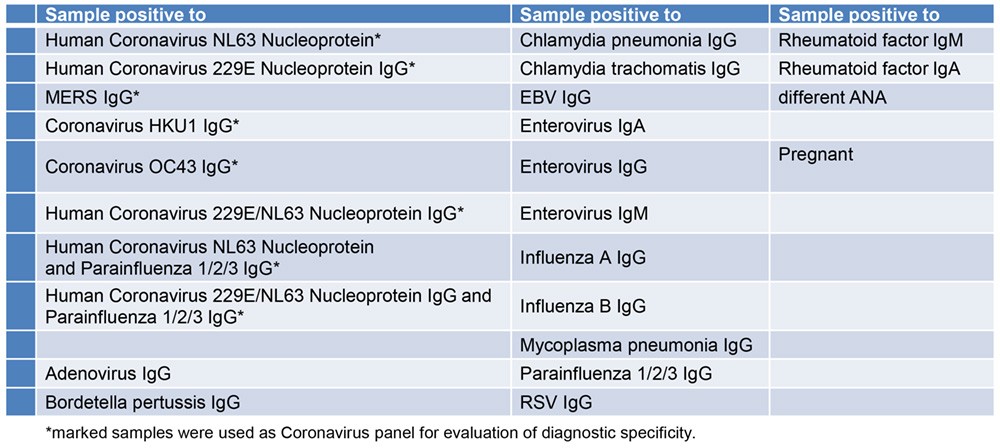
Specificity of Antigen (Cross-Reactivity)
The following samples were tested for cross-reactivity of the assay:
The antigen used for the DRG SARS-CoV-2 (RBD) IgG ELISA† shows no cross-reactivity to the evaluated antibodies.
Objectives of Serological Tests
Diagnosis Support
-
- Support the diagnosis of unclear cases (e.g. PCR positive, but no clinical symptoms)
- Support diagnosis if no PCR is available
Sero-epidemiological studies
-
- Examination of sero-prevalence and seroconversion (IgG)
- Investigation and tracing of transmission paths
- Management of infected people
Identification of a protective immune status
-
- Identification of suitable reconvalescent plasma donors
- Testing the immune status of persons in the health sector
RBD as Antigen
Reasons to use the Receptor Binding Domain (RBD) of the Spike-Protein (S1) as antigen for DRG assays:
Assays detecting RBD can be used to:
- Detect prior virus infection (> 14 days after infection)
- Monitor vaccination success
- Monitor vaccination efficiency (only with quantitative IgG ELISA)
- RBD shows least homology to related coronavirus strains
- RBD protein of the virus mediates virus entry into the cell
- Neutralizing antibodies directed against RBD have been shown to prevent infection of cells
- Many companies (e.g. BioNTech, CureVac, Moderna) develop vaccines based on exposure to RBD

Summary (COVID-19)
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by the newly discovered Severe Acute Respiratory Syndrome Corona Virus 2 (SARS-CoV-2), which was first identified during an outbreak of respiratory illness cases in Wuhan City, China in December 2019 and has been declared an global pandemic by the WHO in March 2020 1I2. SARS-CoV-2 is a positive-sense single-stranded RNA virus and belongs to the Beta coronavirus Genus, which also includes SARS-CoV (2003) and MERS (2012), but also other human coronaviruses like strains HKU1, OC43, NL63, and 229E which cause common cold with normally mild symptoms typically throughout winter month 3.
Explanation
SARS-CoV-2 is predominantly transmitted by droplet infection via coughing or sneezing and through close contact with infected patients. Smear infection through contaminated surfaces seem to play a minor role. Infection mainly occurs in the respiratory tract, but may also be caused through conjunctiva of the eyes. The incubation of COVID-19 ranges between 1-14 days, with the majority of cases manifesting within 4 to 6 days. The clinical manifestations of COVID-19 vary widely from asymptomatic courses to severe pneumonia with lung failure and death. However, in around 80% of cases the symptoms are mild to moderate including fever, cough, respiratory problems, fatigue or temporarily loss of taste. Reported fatality rates increase with age, comorbidities (such as diabetes, cardiovascular or pulmonary disease, chronic liver disease, cancer) and genetic predisposition 4.

The coronavirus spike (S) glycoprotein forms a homotrimeric class I viral fusion complex on the outer envelope of the virion that mediates the binding of the virus to its host cells. Subunit 31 of the spike protein contains a receptor binding domain (RBD) which interacts with the human ACE-2 receptor on the surface of the host cell and initiates the entry of the virus 5-7. Antibodies which are directed against the RBD protein of SARS-CoV-2 are discussed to have neutralizing effects and to protect host cells from viral infection 8,9.
Antibody response to SARS-CoV-2 infection can be detected as early as 3 days after symptom onset and reaches a peak after 2-3 weeks Seroconversion for IgG and IgM occurs simultaneously or sequentially 10,11. In consequence,’ the sensitivity of serological assay increases from the first week of symptom onset (< 40%) to 100% > 15 days after onset12. Duration of time antibodies are present post-infection is not well characterized.
Diagnosis of COVID-19 mainly relies on real-time reverse transcription polymerase chain reaction (RT-PCR) testing of respiratory specimens. However, PCR can’t identify people who went through an infection, recovered, and cleared the virus from their antibodies. Furthermore. chest X-rays, pulmonary computed tomography (CT) scans, and lung ultrasounds are important tools in the early diagnosis of pneumonia in patients with COVID-19 4.
Principles of the Test
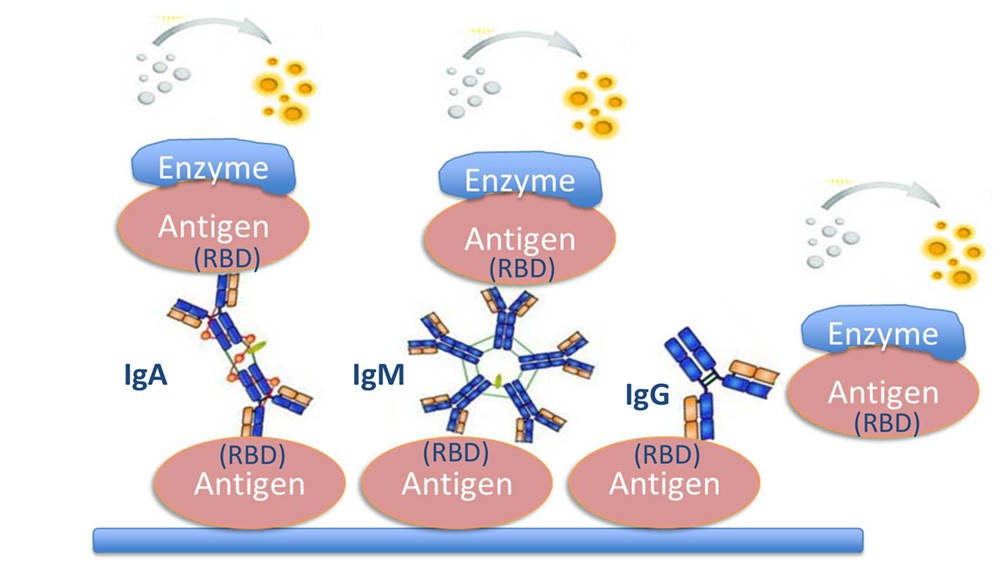
The DRG SARS-CoV-2 (RBD) total Ab ELISA is a solid phase enzyme immunoassay in the one-step antigen capture format.
Microtiter wells as a solid phase are coated with recombinant Receptor-Binding-Domaine (RBD) of the Spike protein antigen
of SARS-CoV-2.
Samples and controls are incubated in the coated wells together with enzyme conjugate (recombinant RBD protein coupled with horseradish peroxidase).
If anti-RBD antibodies are present in the sample, immobilized immune complexes are formed. After a washing step to remove all unbound substances, the solid phase is incubated with substrate solution. The colorimetric reaction is stopped by addition of stop solution, and optical density (OD) of the resulting yellow product is measured.
The intensity of this color is directly proportional to the amount of anti-SARS-CoV-2 RBD antibodies in the patient specimen. Optical density (OD) at 450 nm is read using an ELISA microplate reader. The presence of anti-SARS-CoV-2 RBD antibodies in an individual specimen is determined by comparing the OD values of the specimen to the OD values of the Cut-off Control.
 DRG International Inc: This ELISA is intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection. It can support the diagnosis of COVID-19 disease and supplement direct pathogen detection via RT-PCR or pulmonary CT-Scans. In addition, serology can help to gather epidemiological information on the disease prevalence.
DRG International Inc: This ELISA is intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection. It can support the diagnosis of COVID-19 disease and supplement direct pathogen detection via RT-PCR or pulmonary CT-Scans. In addition, serology can help to gather epidemiological information on the disease prevalence.
How do I get the DRG SARS-CoV-2 (RBD) total Ab ELISA at my facility?
Click the ‘Shop Now‘ button to order directly from our eStore or click here to contact us for more information, and connect with a regional DRG International Sales Representative
† – “Due to Regulatory Restrictions, sales of these products may not be available in your territory. The SARS kits are CE/IVD in the EU”
Disclaimer: The information contained in this communication from the sender is confidential. It is intended solely for use by the recipient and others authorized to receive it. If you are not the intended recipient, you are hereby notified that any disclosure, copying, use, or distribution of the information included in this email is prohibited and may be unlawful.
References / Literature
- Ji T. et al. Detection of Covid-19: A review of the current literature and future perspectives. Biosensors and Bioelectronics, 2020 166: 112455
- World Health Organization. Coronavirus disease 2019 (COVID.19) situation report-85, Data as received by WHO from national authorities by 10:00 CET, 14 April 2020.
Accessed April 14, 2020. https//www.who.intidocs/default-sourcelcoronaviruse/situation-reports/20200414-sitrep-85-covid-19. - Zhong N et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong People’s Republic of China February 2003. Lancet 2003 362-1363.1358
- da Silva SJR et al. Clinical and laboratory diagnosis of SARS-CoV-2, the virus causing COVID-19. ACS Inf Dis. August 2020; https:/dx.doi.org/10.1021/acsinfecdis.Oc00274.
- Brielle ES, Schneidman-Duhovny D, and Linial M. The SARS-CoV-2 exerts a distinctive strategy for interacting with the ACE2 human receptor. Viruses. 2020 12(5):497.
- Gui M et al. Cryo-electron microscopy structures of the SARS-CoV spike glycoprotein reveal a prerequisite conformational state for receptor binding
Cell Research. 2017 27:119-129. - Turonova B et al. In situ structural analysis of SARS-CoV-2 spike reveals flexibility mediated by three hinges. Science, 2020 10.1126/science.atbd.5223.
- Premkumar L et al. The receptor binding domain of the viral spike protein is an immunodominant and highly specific.
Science Immunology 2020 10.1126/sciimmunol.abc8413. - Robbiani DF et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020 584:437-456
- Kontou Pl et al. Antibody tests in detecting SARS-CoV-2 infections: a meta-analysis. Diagnostics. 2020 10:319-34.
- Long QX et al. Convergent antibody responses to SARS-CoV-2 in patients with COVID-19, Nature Medicine 2020 26-845-848
- Zhao J et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Inf Dis. 2020 Accepted manuscript doi: 10.1093/cid/ciaa344