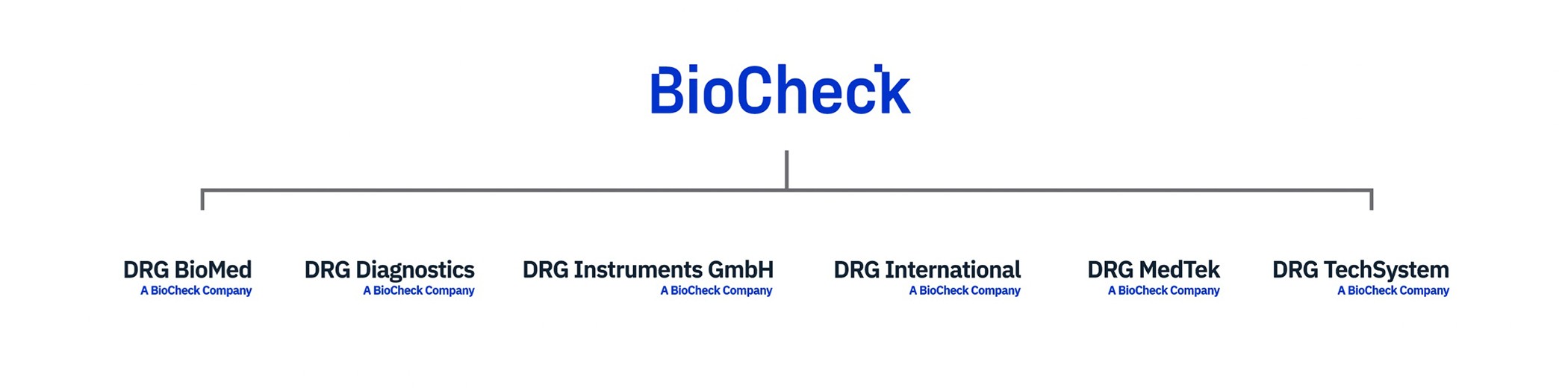
BioCheck, Inc. is the parent company of DRG. BioCheck, located in South San Francisco, California, USA, was found in April 1997. BioCheck acquired DRG International with offices in USA, Germany, Russia, Poland, and Czech Republic in August 2021. BioCheck designs, develops, and manufactures high-quality, In vitro diagnostic immunoassay devices, for the worldwide biomedical, pharmaceutical, and scientific research markets. Product categories include Tumor Markers, Cardiac Markers, Steroids, TORCH, Infectious Diseases, Thyroids, and Fertility. BioCheck also offers antibody purification and conjugation services.
BioCheck is an ISO 13485:2016 certified and U.S. FDA 21 CFR Part 820 compliant company that provides quality products and is committed to best-in-class customer care. Together with the DRG subsidiaries, BioCheck’s goal is to provide timely information so treatment can be implemented earlier, and effectiveness can be analyzed sooner to better the long-term outcomes or better life.
DRG International, Inc. is a leading manufacturer of clinical diagnostic and research ELISAs with distributors in over 110 countries. DRG is also the manufacturer of the DRG:HYBRiD-XL(r), a fully automated analyzer for Immunoassays and Clinical Chemistry. Founded in 1970, DRG International, Inc. provides a complete range of products and services to the diagnostic and research communities. DRG International’s global headquarters is conveniently situated in Springfield, New Jersey. Only 20 miles from New York City and 80 miles from Philadelphia, PA; DRG International, Inc. is in the heart of the Tri-State Area.
Quality – DRG International, Inc. operates in accordance with the FDA 21 CFR 820 Quality System Regulation as well as the ISO 13485:2016 standard. DRG maintains certifications in both ISO 13485:2016 and MDSAP (Medical Device Single Audit Program) by TUV Rheinland.
Sales – The majority of DRG sales are in immunodiagnostics worldwide, however, DRG is also an authorized distributor of CHROMagar chromogenic medium within the United States. With our MDSAP certification, as well as over 200 FDA-approved products and over 75 products registered with Health Canada, we are looking to expand our reach throughout North America and globally. Strong markets for DRG products are throughout Europe, Poland, Russia, the US, the Middle East, Asia- Pacific, China, and South America.
Founder – Dr. Cyril E. Geacintov (1930 – 2017)
President & CEO – Roy Paxton Yih
Mission Statement
DRG International, Inc., is committed to providing the medical and research community with cutting edge, easy-to-use and effective diagnostic devices. Founded in 1970, DRG International is constantly looking towards the future in order to continue to produce and distribute the highest-quality products worldwide.
Office Locations
Click a location on the map below to receive more information about the selected office.
| Title | Address | Description |
|---|---|---|
UNITED STATES | 425 Eccles Ave, South San Francisco, CA 94080, USA | BioCheck, Inc. 425 Eccles Avenue South San Francisco, CA 94080 USA Tel: +1-650-573-1968 |
UNITED STATES | 841 Mountain Ave, Springfield Township, NJ 07081, USA | DRG International, Inc. Global Headquarters Tel: +1-973-564-7555 |
GERMANY | Frauenbergstraße 18, 35039 Marburg, Germany | DRG Instruments GmbH, R&D and Production Tel: +49 (0)-6421/1700-0 |
RUSSIA - Moscow | Moscow, Russia, 117218 | DRG Techsystems, ZAO Tel: +7 (499)-277-07-20 |
RUSSIA - St. Petersburg | St Petersburg, Russia, 194156 | DRG Biomed, OOO Tel: +7 (812)-492-5693/492-5793 |
POLAND | Wita Stwosza 24, 02-661 Warszawa, Poland | DRG MEDTEK Tel: +48-(22)-847-82-44/83-75 |
CZECH REPUBLIC | Šumavská 33, 602 00 Brno-střed, Czechia | DRG Spol. S.R.O. Tel/Fax: +00420-532-091-339 |
Philanthropic/Board Memberships
Parasites & Helicobacter Pylori in Egyptian Children | Calprotectin- DRG:HYBRiD-XL
Parasites & Helicobacter Pylori in Egyptian Children with or without Diabetes with Gastrointestinal Manifestations & High Calprotectin Level
Estimating the Burden of Iron Deficiency
Background: Iron deficiency (ID) is a major public health burden in African children and accurate prevalence Abstract Background and Objective Iron deficiency (ID) is a major public health burden in African children and accurate prevalence estimates are...
Early postnatal hypoferremia in low birthweight, preterm babies
Early postnatal hypoferremia in low birthweight and preterm babies: A prospective cohort study in hospital-delivered Gambian neonates Abstract Background Neonates, particularly those born preterm (PTB) and with low birthweight (LBW), are especially susceptible...
Get the Latest News & Updates
Join our email list to get the latest news and updates from DRG International. Unsubscribe anytime.