Salivary ELISA Kits
Salivary ELISA Kits
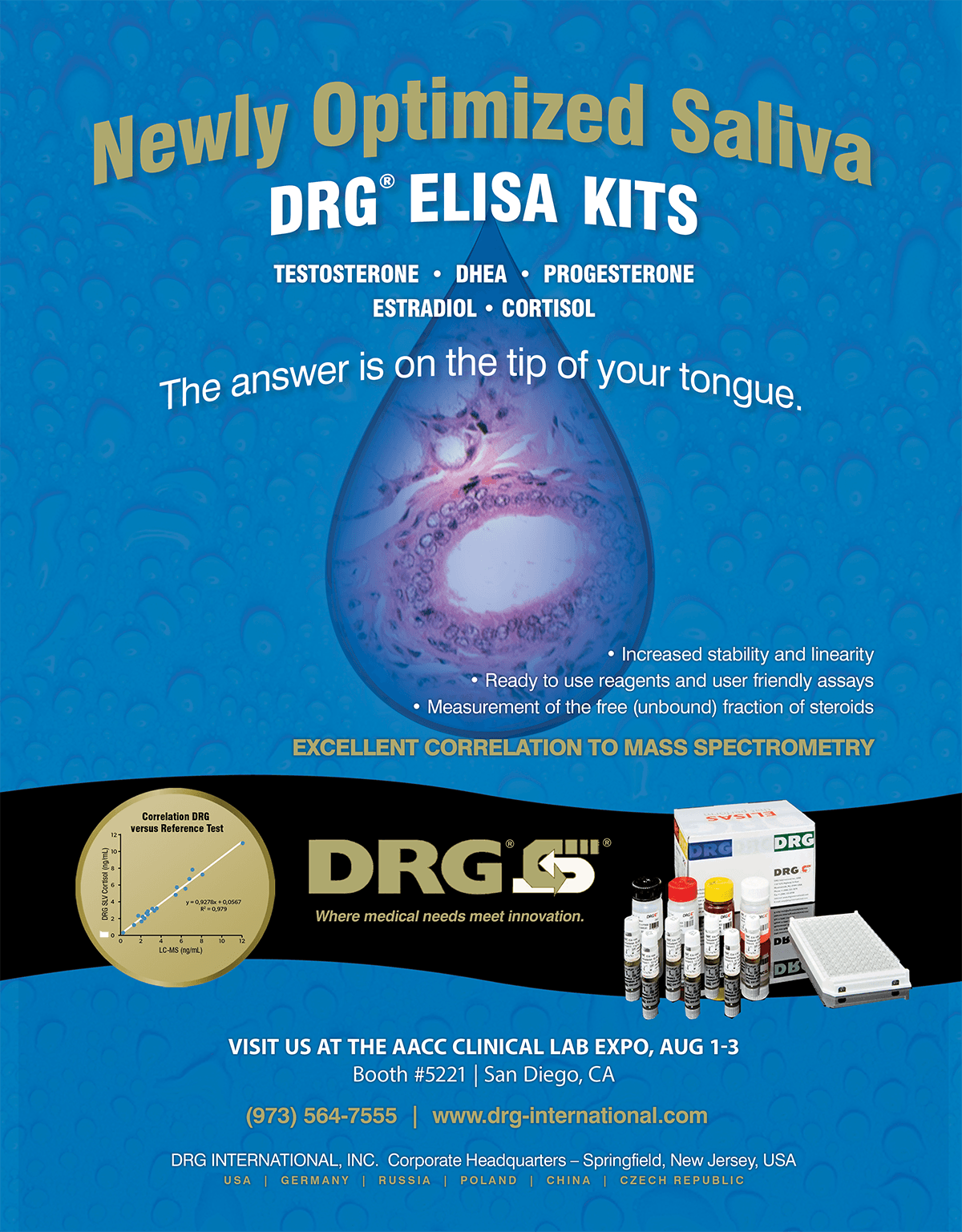
Salivary ELISA Kits are easy-to-use, non-invasive sampling test kits designed for the measurement of the free (unbound) fraction of steroids. These tests allow for simple, user-friendly measurement of hormone profiles with reliable measurement of steroids in saliva samples without further pre-treatment.
Salivary ELISA Assays include:
Optimized Salivary Cortisol ELISA RUO (SLV-2930R)
Industry Leading Accuracy with Kit Components Calibrated to Mass Spectrometry
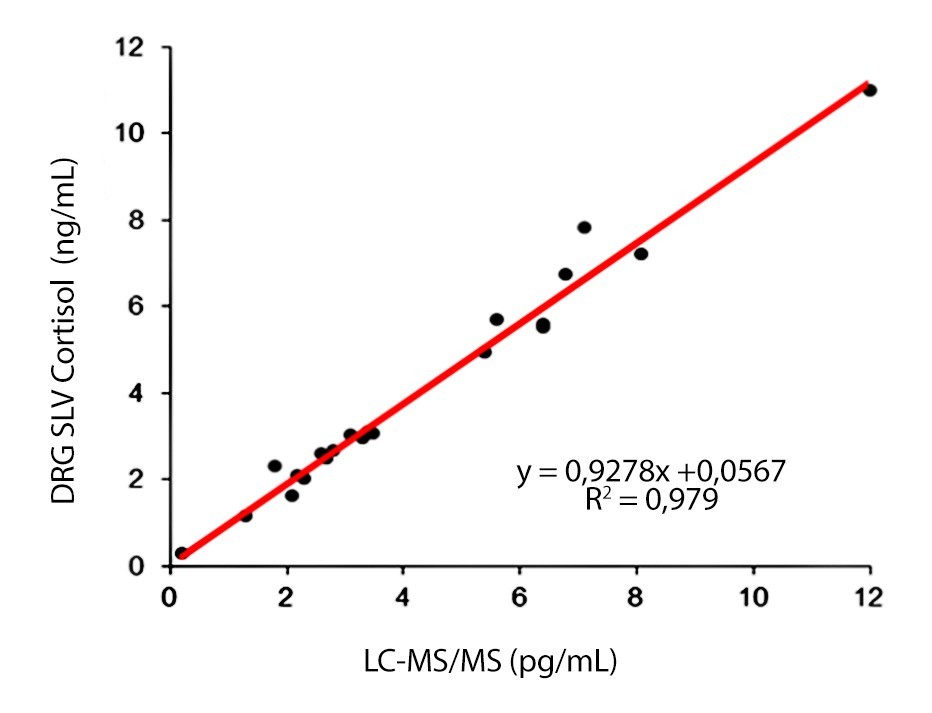
Correlation DRG vs. Reference Test
Advantages of the New Cortisol Saliva Kit
- No change in assay procedure
- Monoclonal antibody coated on microtiterplate
- Reagent stability increased
- Assay Dynamic Range (0.09 – 30 ng/mL)
- Reference values are more detailed with differentiation between morning, noon and evening
- New range 0.2 – 19.80 ng/ml, compared to 1.2 – 14.7 ng/ml of the old version
- Very good correlation to LC-MS (r2=0,979) and to competitor´s ELISA (r2=0,989)
- Conversion factor: Result old version x 0,48 = Result new version
Excellent Correlation to Mass Spectrometry
Optimized Salivary Progesterone HS Assay 96 well MPTL ELISA
(SLV-5911)
Industry Leading Accuracy with Kit Components Calibrated to Mass Spectrometry
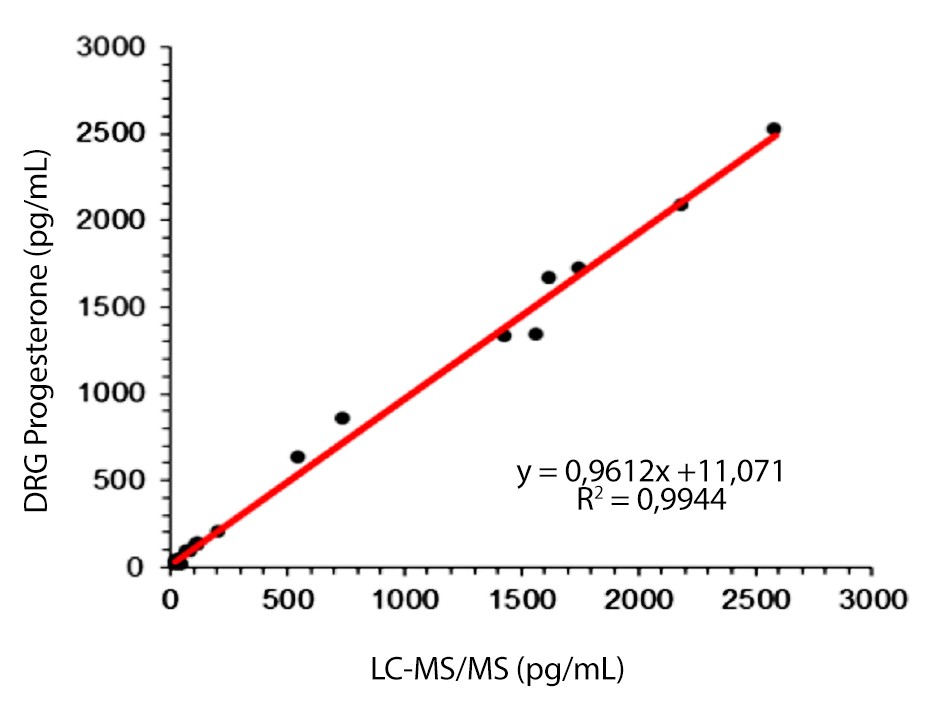
SLV Progesterone
Advantages of the Progesterone Saliva Kit
- Good reagent stability
- Assay Dynamic Range 1.1 – 2,400 pg/mL
- High sensitivity (1 pg/mL)
- Short incubation time (60/15 min)
- Reference values with detailed differentiation of menstrual cycle
- Very good correlation to LC-MS/MS mass spectrometer (r2=0,994)
Excellent Correlation to Mass Spectrometry
DRG Saliva Assay Advantage
Clear Advantages
Simple, painless collection
Simple and patient-friendly measurement of hormone profiles (Diurnal-, monthly profiles)
Relatively inexpensive form of testing
Collection
Non-invasive sampling, can be performed also by patients
Easy sampling in babies and kids (babies need special sampling devices)
Study special populations where blood collection is a problem; handicap, elderly, anxious patients
Serum-Saliva Correlation
Results provide good correlation to comparable serum testing
Sensitivity
Provides accurate, low level detection
Measurement of the free (unbound) fraction of steroids
Measurement of steroids and other nonpeptide hormones
Clinical Applications
Diagnosis of systemic/local conditions
Obstetrics & Gynecology
Nutrition and Natural Medicine
Sports Medicine
Drug Monitoring
Forensics
News and Updates
BioCheck Inc Acquires DRG International Inc
Transformative deal significantly increases IVD development, manufacturing and sales channel for novel ELISA and Chemiluminescent immunoassay platforms.
Parasites & Helicobacter Pylori in Egyptian Children | Calprotectin- DRG:HYBRiD-XL
Parasites & Helicobacter Pylori in Egyptian Children with or without Diabetes with Gastrointestinal Manifestations & High Calprotectin Level
Cortisol & Adrenal Androgens as Independent Predictors
Cortisol and adrenal androgens as independent predictors of mortality in septic patients. Abstract Background and Objective To determine the prognostic value of cortisol, Dehydroepiandrosterone (DHEA) and Dehydroepiandrosterone-sulfate (DHEAS), together with their...